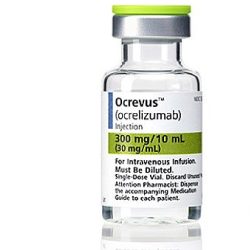
Ocrevus (Ocrelizumab)
- Medicine Name: Ocrevus
- Generic Name: Ocrelizumab
- Dosage Form & Strength: Injection: 300 mg/10 mL (30 mg/mL) in a single-dose vial
- Manufactured By: Genentech, Inc.
Medical Uses
Ocrevus is a CD20-directed cytolytic antibody used for the treatment of:
Relapsing forms of multiple sclerosis (RMS), to include clinically isolated syndrome, active secondary progressive disease and relapsing-remitting disease, in adults.
Primary progressive multiple sclerosis (PPMS), in adults.
Recommended Dosage: Hepatitis B virus and quantitative serum immunoglobulin screening are required prior to the first dose. Patients should be pre-medicated with methylprednisolone (or an equivalent corticosteroid) and an antihistamine (e.g., diphenhydramine) before each infusion. Administer Ocrevus 300 mg/10 mL injection by intravenous infusion.
Start dose: 300 mg administered as an intravenous infusion, followed two weeks later by a second 300 mg administered as an intravenous infusion.
Subsequent doses: 600 mg administered as an intravenous infusion every 6 months.
Must be diluted prior to administration. Monitor patients precisely throughout and for at least 60 minutes after the infusion.
If any planned infusion is missed, administer as promptly as possible; do not wait until the next scheduled dose. It is advisable to reset the dose schedule to administer the next sequential dose six months after the missed dose is administered. Doses must be separated by at least five months.
Warning & Precautions
- Ocrevus treatment can cause infusion reactions, which can include rash, urticaria, pruritus, throat irritation, erythema, bronchospasm, dyspnea, pharyngeal or laryngeal edema, flushing, fatigue, hypotension, headache, nausea, dizziness, and anaphylaxis. Observe those treated with Ocrevus for infusion reactions during the infusion and for at least 1-hour after completion of the infusion.
- Ocrelizumab may increase the probability for upper/lower respiratory tract infections, skin infections, and herpes-related infections. Therapy was not linked with an increased risk of severe infections in patients with MS. Delay Ocrelizumab administration in those with an active infection until the infection is resolved.
- Severe cases of infections caused by varicella zoster virus, herpes simplex virus, including central nervous system infections (meningitis and encephalitis), intraocular infections, and disseminated skin and soft tissue infections, may occur in multiple sclerosis (MS) patients receiving ocrevus 300mg/10 mL injection.
- Serious herpes virus infections may occur at any time throughout treatment with Ocrelizumab. A few cases can be life-threatening. In case serious herpes infections occur, Ocrelizumab should be interrupted/discontinued or withheld until the infection has resolved, and apt treatment should be administered.
- Hepatitis B reactivation may occur in MS patients treated with Ocrelizumab. Hepatic failure, fulminant hepatitis and death caused by HBV reactivation may occur in those treated with anti-CD20 antibodies. HBV screening should be performed in all patients prior to the initiation of treatment with Ocrelizumab. Do not administer this medicine to those with active HBV confirmed by positive results for HBsAg and anti-HB tests.
- Use all immunizations as per the immunization guidelines at least four weeks before initiation of Ocrelizumab 300mg/10 mL for live or live-attenuated vaccines and, whenever possible, at least 14 days prior to initiating Ocrelizumab for non-live vaccines. Ocrelizumab may be responsible for interfering with the effectiveness of non-live vaccines.
- Decreased immunoglobulin levels may occur with Ocrelizumab treatment. Quantitative serum immunoglobulins levels should be monitored during therapy and after interruption of therapy. Consider discontinuing Ocrelizumab therapy in those with serious opportunistic or recurrent severe infections, and in case prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
- Immune-mediated colitis, which can present as a severe and acute-onset form of colitis, may occur in those on ocrelizumab treatment. Evaluate the patients for immune-mediated colitis during therapy, and assess promptly if signs/symptoms that may indicate immune-mediated colitis, such as new or persistent diarrhea or any other gastrointestinal signs/symptoms, occur.
- Females of childbearing age should use effective contraception while on Ocrelizumab and for six months after the last infusion. If patients are pregnant or plan to become pregnant while on Ocrelizumab, they should inform their health specialist.
Documentation & Availability
What documents are required to import OCREVUS to India?
OCREVUS (Ocrelizumab) injection can be imported by patients or government hospitals on the name of the patients only.
The following documentation is required to import the product:
- A valid prescription from a qualified doctor.
- Patient’s diagnostic reports.
- Patient’s ID proof (issued by the Government of India).
How is the order confirmed?
The order will be confirmed only after the receipt of:
- A valid prescription from a doctor.
- Import permit if applicable.
Is OCREVUS available in India?
OCREVUS (Ocrelizumab injection) is a (prescription drug, prescription medication or prescription medicine) pharmaceutical drug that legally requires a medical prescription to be dispensed.
S S Healthcare (S S Healthcare) helps import cancer medicines on the named patient supply (NPS). Indian Pharma Network is the facilitator providing input
- On availability in India (Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore and Pune etc.)
- Medicine Price.
- Finding Genuine and reliable source from Canada, Europe, USA and Australia
- Ensuring 100% transparency.
OCREVUS can be made available to patients, doctors and hospitals at Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore, Srinagar, Jammu, Jaipur, Chandigarh, Ludhiana, Noida, Gurgaon, Lucknow and Pune and other cities in India. The order will be confirmed only after the receipt of a valid prescription from the doctor and import permit.
S S Healthcare (S S Healthcare) can facilitate the supply of OCREVUS (prescription medicines) to all locations in the world and in India after fulfilling the legal requirement (if applicable).
Please contact +91-7575883015 | Number: +91-7575883015 or write us at sshealthcare1012@gmail.com for OCREVUS 300 mg/10 mL (30 mg/mL)- Ocrelizumab price in India.
We take guarantee of quality and delivery anywhere in the world as per the buyer’s requirements.
Sourcing & Delivery
S S Healthcare is able to source the OCREVUS (Cancer Treatment Medicines) from across the globe, and has the ability to supply. S S Healthcare offers its customers worldwide access to the best available treatment.
S S Healthcare is able to dispense any valid prescription in the shortest possible time. All prescriptions are dispensed and checked by registered pharmacists and dispatched to the Patient’s address only from New Delhi, India.
FAQ
What is the Generic Name for the trade name drug Ocrevus®?
Ocrelizumab is Generic Name for the trade name drug Ocrevus®.
What is the Manufacturer Name of Ocrevus®?
Ocrevus® is manufactured by Genentech, Inc.
Is Ocrevus® approved by the FDA?
Yes, Ocrevus® is approved by the FDA. Date of first/initial approval: March 28, 2017.
Where can I get Ocrevus® at the best price in India?
To get the best Ocrevus price in India, kindly contact S S Healthcare (a WHO-GDP & ISO 9001:2008 authorized Company). A valid medical prescription is required while buying Ocrevus®.
What is the dosage and form of Ocrevus® supplied?
Ocrevus is supplied as Injection: 300 mg/10 mL (30 mg/mL) in a single-dose vial for intravenous administration.
What are the most common side effects with Ocrevus®?
The most common side effects with Ocrevus® are:
- RMS: infusion reactions and upper respiratory tract infections.
- PPMS: upper respiratory tract infections, skin infections, infusion reactions, and lower respiratory tract infections.
How much does Ocrevus® cost in India?
The ocrevus cost in India is less and can vary. In order to procure this medication authentically, you can Call or WhatsApp +91-7575883015 or send mail to sshealthcare1012@gmail.com.
What are the storage conditions of Ocrevus®?
Store the vials of Ocrevus at 2°C to 8°C (36°F to 46°F) in the outer carton in order to protect from the light. Do not freeze or shake.
Is it safe to buy Ocrevus® online from India?
Yes, You can buy Ocrevus injection online in India at the best price from the S S Healthcare if Ocrevus® has not been approved or is not available in your country.
What are the Highlights of prescribing information for Ocrevus®?
Click Here to download full ocrevus prescribing information.
Contact Patient Support
If you have any questions or need any help, contact our Patient Support Team. We will get in touch with you within 24 hours from Monday to Friday between 9:00 and 10:00 CET.
Email Address
Sshealthcare1012@gmail.com
+91 7575883015
Landline
079 48916267
Disclaimer
All Trademarks and Brands that appear on the website belong to their respective owners, and S S Healthcare does not lay any claim on them. We only provide information.