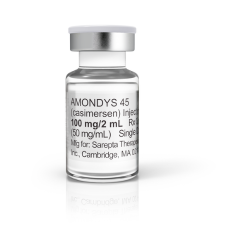
Amondys 45 (Casimersen)
- Medicine Name: Amondys 45
- Generic Name: Casimersen
- Dosage Form & Strength: Injection: 100 mg/2 mL in a Single-Dose Vial
- Manufactured By: Sarepta Therapeutics, Inc.
Medical Uses
Amondys 45 is an antisense oligonucleotide used for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping.
Recommended Dosage: The recommended dosage is 30 milligrams per kilogram administered once weekly as a 35 to 60-minute intravenous infusion via an in-line 0.2 micron filter.
In case a dose of Amondys 45 (Casimersen Injection) is missed, it may be administered as promptly as possible after the scheduled dose.
Warning & Precautions
- Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio (UPCR) needs to be measured prior to starting Casimersen therapy. Consider measurement of glomerular filtration rate before initiating Casimersen treatment.
- During treatment with injection casimersen 10 mg, monitor urine dipstick every month, and serum cystatin C and urine protein-to-creatinine ratio (UPCR) every three months.
- Nephrotoxicity may occur with medicines similar to Amondys 45. Patients need to be monitored for kidney toxicity during treatment. Obtain the urine sample before Amondys 45 infusion or at least 48 hours after infusion.
Documentation & Availability
What documents are required to import AMONDYS 45 to India?
AMONDYS 45 (casimersen) injection can be imported by patients or government hospitals on the name of the patients only.
The following documentation is required to import the product:
- A valid prescription from a qualified doctor.
- Patient’s diagnostic reports.
- Patient’s ID proof (issued by the Government of India).
How is the order confirmed?
The order will be confirmed only after the receipt of:
- A valid prescription from a doctor.
- Import permit if applicable.
Is AMONDYS 45 available in India?
AMONDYS 45 (casimersen injection) is a (prescription drug, prescription medication or prescription medicine) pharmaceutical drug that legally requires a medical prescription to be dispensed.
S S Healthcare (S S Healthcare) helps import cancer medicines on the named patient supply (NPS). Indian Pharma Network is the facilitator providing input
- On availability in India (Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore, Pune etc.)
- Medicine Price.
- Finding Genuine and reliable sources from the USA, Canada, Europe, and Australia
- Ensuring 100% transparency.
AMONDYS 45 can be made available to patients, doctors and hospitals at Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore, Srinagar, Jammu, Jaipur, Chandigarh, Ludhiana, Noida, Gurgaon, Lucknow and Pune and other cities in India. The order will be confirmed only after the receipt of a valid prescription from the doctor and import permit.
S S Healthcare (S S Healthcare) can facilitate the supply of AMONDYS 45 (prescription medicines) to all locations in the world and in India after fulfilling the legal requirement (if applicable).
Please contact +91-7575883015 | Toll-Free No: +91-7575883015 or write us at sshealthcare1012@gmail.com for Amondys 45 injection price in India.
We take guarantee of quality and delivery anywhere in the world as per the buyer’s requirements.
Sourcing & Delivery
S S Healthcare is able to source the AMONDYS 45 (Cancer Treatment Medicines) from across the globe, and has the ability to supply. S S Healthcare offers its customers worldwide access to the best available treatment.
S S Healthcare is able to dispense any valid prescription in the shortest possible time. All prescriptions are dispensed and checked by registered pharmacists and dispatched to the Patient’s address only from New Delhi, India.
FAQ
What is the Generic Name for the trade name drug Amondys 45®?
Casimersen is Generic Name for the trade name drug Amondys 45®.
What is the Manufacturer Name of Amondys 45®?
Amondys 45® is manufactured by Sarepta Therapeutics, Inc.
Is Amondys Injection approved by the FDA?
Yes, amondys injection is approved by the FDA. Date of approval: February 25, 2021.
What is Amondys 45®?
Amondys 45® is approved by the FDA to treat patients with Duchenne muscular dystrophy (DMD) who have a confirmed mutation of the dystrophin gene that can be treated by skipping exon 45.
What is the dosage and form of Amondys 45® supplied?
Amondys 45® is supplied as Injection: 100 mg/2 mL (50 mg/ mL) solution in a single-dose vial for intravenous administration.
How long will my Infusion last?
It usually takes about 35-60 minutes to intravenously infuse the medicine, via an in-line 0.2 micron filter.
What are the most common side effects due to Amondys 45®?
Most common side effects due to Amondys 45® include: cough, pyrexia, upper respiratory tract infection, headache, arthralgia, and oropharyngeal pain.
How much does Amondys 45® cost in India?
Amondys 45 cost in India is very reasonable. In order to procure this DMD medicine authentically, you can call or WhatsApp +91-7575883015 or send mail to sshealthcare1012@gmail.com.
What are the storage conditions of Amondys 45®?
Store Amondys 45 vials at 2°C to 8°C (36°F to 46°F). Do not freeze. Store in the original carton until ready for use to protect from light.
What are the Highlights of prescribing information for Amondys 45®?
Click Here to download full Amondys 45 prescribing information
Contact Patient Support
If you have any questions or need any help, contact our Patient Support Team. We will get in touch with you within 24 hours from Monday to Friday between 9:00 and 10:00 CET.
Email Address
Sshealthcare1012@gmail.com
+91 7575883015
Landline
079 48916267
Disclaimer
All Trademarks and Brands that appear on the website belong to their respective owners, and S S Healthcare does not lay any claim on them. We only provide information.