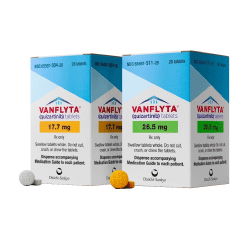
Vanflyta (Quizartinib)
- Medicine Name: Vanflyta
- Generic Name: Quizartinib Dihydrochloride
- Dosage Form & Strength: Tablets: 17.7 mg or 26.5 mg.
- Manufactured By: Daiichi Sankyo, Inc.
Medical Uses
Vanflyta (Quizartinib) is a kinase inhibitor used in conjunction with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, to treat adults with newly diagnosed AML (acute myeloid leukemia) that is FLT3-ITD-positive.
Recommended Dosage: The recommended dose of Vanflyta tablet is administered orally with or without food at around the same time each day. Swallow tablets whole. Do not crush, cut, or chew them. The recommended dose is as follows:
- Induction: 35.4 mg taken orally once daily on Days 8 to 21 of “7 + 3” (cytarabine [100 or 200 mg/m2 per day] on Days 1 to 7 plus daunorubicin [60 mg/m2 per day] or idarubicin [12 mg/m2 per day] on Days 1 to 3) and on Days 8-21 or 6-19 of an optional second induction (“7 + 3” or “5 + 2” [5 days cytarabine plus 2 days idarubicin or daunorubicin], respectively),
- Consolidation: 35.4 mg taken orally once daily on Days 6 to 19 of high‑dose cytarabine (1.5 to 3 g/m2 every 12 hours on Days 1, 3 & 5) for up to 4 cycles, and
- Maintenance: 26.5 mg taken orally once daily on Days 1 to 14 and 53 mg once daily, thereafter, for up to thirty-six 28-day cycles.
If a dose is vomited, do not take a replacement dose; wait until the very next scheduled dose is due. If a dose is missed or not taken at the usual time, administer the dose as quickly as possible on the same day and return to the usual schedule the following day. The patient must not take a couple of doses on the same day.
Warning & Precautions
- Vanflyta (Quizartinib) is a kinase inhibitor used in conjunction with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, to treat adults with newly diagnosed AML (acute myeloid leukemia) that is FLT3-ITD-positive.
- Recommended Dosage: The recommended dose of Vanflyta tablet is administered orally with or without food at around the same time each day. Swallow tablets whole. Do not crush, cut, or chew them. The recommended dose is as follows:
- Induction: 35.4 mg taken orally once daily on Days 8 to 21 of “7 + 3” (cytarabine [100 or 200 mg/m2 per day] on Days 1 to 7 plus daunorubicin [60 mg/m2 per day] or idarubicin [12 mg/m2 per day] on Days 1 to 3) and on Days 8-21 or 6-19 of an optional second induction (“7 + 3” or “5 + 2” [5 days cytarabine plus 2 days idarubicin or daunorubicin], respectively),
- Consolidation: 35.4 mg taken orally once daily on Days 6 to 19 of high‑dose cytarabine (1.5 to 3 g/m2 every 12 hours on Days 1, 3 & 5) for up to 4 cycles, and
- Maintenance: 26.5 mg taken orally once daily on Days 1 to 14 and 53 mg once daily, thereafter, for up to thirty-six 28-day cycles.
- If a dose is vomited, do not take a replacement dose; wait until the very next scheduled dose is due. If a dose is missed or not taken at the usual time, administer the dose as quickly as possible on the same day and return to the usual schedule the following day. The patient must not take a couple of doses on the same day.
Documentation & Availability
What documents are required to import VANFLYTA® to India?
VANFLYTA® (quizartinib) tablets can be imported by patients or government hospitals on the name of the patients only.
The following documentation is required to import the product:
- A valid prescription from a qualified doctor.
- Patient’s diagnostic reports.
- Patient’s ID proof (issued by the Government of India).
How is the order confirmed?
The order will be confirmed only after the receipt of:
- A valid prescription from a doctor.
- Import permit if applicable.
Is VANFLYTA® available in India?
VANFLYTA® (quizartinib tablets) is a (prescription drug, prescription medication, or prescription medicine) pharmaceutical drug that legally requires a medical prescription to be dispensed.
S S Healthcare (S S Healthcare) helps import cancer medicines on the named patient supply (NPS). Indian Pharma Network is the facilitator providing input
- On availability in India (Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore and Pune etc.)
- Medicine Price.
- Finding Genuine and reliable source from Canada, Europe, USA and Australia
- Ensuring 100% transparency.
VANFLYTA® can be made available to patients, doctors, and hospitals in Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore, Srinagar, Jammu, Jaipur, Chandigarh, Ludhiana, Noida, Gurgaon, Lucknow, and Pune and other cities in India. The order will be confirmed only after the receipt of a valid prescription from the doctor and an import permit.
S S Healthcare (S S Healthcare) can facilitate the supply of VANFLYTA® (prescription medicines) to all locations in the world and in India after fulfilling the legal requirement (if applicable)
Please contact +91-7575883015 | Number: +91-7575883015 or write us at sshealthcare1012@gmail.com for Vanflyta price in India.
We take to guarantee quality and delivery anywhere in the world as per the buyer’s requirements.
Sourcing & Delivery
S S Healthcare is able to source the VANFLYTA® (Cancer Treatment Medicines) from across the globe and has the ability to supply. S S Healthcare offers its customers worldwide access to the best available treatment.
S S Healthcare is able to dispense any valid prescription in the shortest possible time. All prescriptions are dispensed and checked by registered pharmacists and dispatched to the Patient’s address only from New Delhi, India.
FAQ
What is the Generic Name for the trade name drug Vanflyta®?
Quizartinib is a Generic Name for the trade name drug Vanflyta®.
What is the Manufacturer’s Name of Vanflyta®?
Vanflyta® is manufactured by Daiichi Sankyo, Inc.
Is Vanflyta® approved by the FDA?
Yes, Vanflyta® is approved by the FDA. Date of approval: July 20, 2023.
What is the dosage and form of Vanflyta® supplied?
Vanflyta® is supplied as Tablets: 17.7 mg or 26.5 mg for oral administration.
What are the most common side effects of Vanflyta®?
The most common side effects of Vanflyta® are diarrhea, calcium decreased, mucositis, abdominal pain, upper respiratory tract infection, sepsis, nausea, neutropenia, vomiting, and headache.
How much does Vanflyta® cost in India?
The cost of Vanflyta in India can vary. In order to procure this AML medication legally, you can Call or WhatsApp +91-7575883015 or send mail to sshealthcare1012@gmail.com.
What are the storage conditions of Vanflyta®?
Store the film-coated tablets of Vanflyta® at 20 to 25°C (68 to 77°F); excursions permitted from 15-30°C (59-86°F).
Is it safe to buy Vanflyta® online in India?
Yes, one can buy Vanflyta online in India at the lowest price from the S S Healthcare if the medicine Vanflyta® is not (yet) registered or is available in your country. We can help facilitate the supply of Vanflyta® through legal channels.
Contact Patient Support
If you have any questions or need any help, contact our Patient Support Team. We will get in touch with you within 24 hours from Monday to Friday between 9:00 and 10:00 CET.
Email Address
Sshealthcare1012@gmail.com
+91 7575883015
Landline
079 48916267
Disclaimer
All Trademarks and Brands that appear on the website belong to their respective owners, and S S Healthcare does not lay any claim on them. We only provide information.